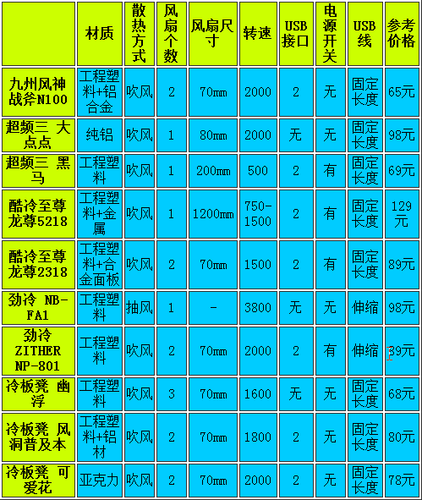
美国Myriad genetics生物公司19日宣布,该公司的BRACAnalysis CDx已经通过FDA批准用于阿斯利康的Lynparz优艾设计网_电脑技术a(olaparib)
ty_v紫v 2021-06-10 04:33 优艾设计网_PS问答
在FDA官网上的关于FDA approves Lynparza to treat advanced ovarian cancer公告显示,BRACAnalysis CDx已经是通过FDA批准用于阿斯利康的Lynparza(olaparib)—一种与BRCA基因有关的晚期卵巢癌新药的伴随诊断。
The FDA approved Lynparza with a genetic test called BRACAnalysis CDx, a companion diagnostic that will detect the presence of mutations in the BRCA genes (gBRCAm) in blood samples from patients with ovarian cancer. The BRCA genes are involved with repairing damaged DNA and normally work to suppress tumor growth. Women with mutations resulting in defective BRCA genes are more likely to get ovarian cancer, and it is estimated that 10 to 15 percent of all ovarian cancer is associated with these hereditary BRCA mutations.
ty_138546045 2021-06-10 04:40 优艾设计网_设计客
BRACAnalysis CDx获FDA批准用于晚期卵巢癌药物伴随诊断,是一种检测卵巢癌患者全血样本基因组的BRCA1/2基因突变的体外诊断分析系统。该检测系统经过Myriad genetics公司在盐湖城实验室的专业验证,并且,临床实验结果显示该检测可以有效鉴定卵巢癌患者的BRCA突变。





 加载中,请稍侯......
加载中,请稍侯......
精彩评论